As the delta virus surges across the nation, hospitalizations have also soared and reached 100,000. This high level has been seen after little over six months as the last time there was such a high rate was on January 30, when vaccines were not easily available.
According to a Washington Post data base, the South has a higher rate of hospitalizations due to COVID-19, when compared with the rest of the nation. The maximum number of hospitalizations is in Florida. More than 17,000 people are in hospitals in the Sunshine State. Texas follows close behind with more than 14,000 hospitalizations. However, deaths are down at 1,100 when compared to the daily average of 3,100 deaths in January.
As Southern states remove mask mandates in schools, the number of pediatric hospitalizations is also on the increase. The current number of hospitalizations is 2,100 nationally which is higher than the number of hospitalizations in August 2020 which was 2000.
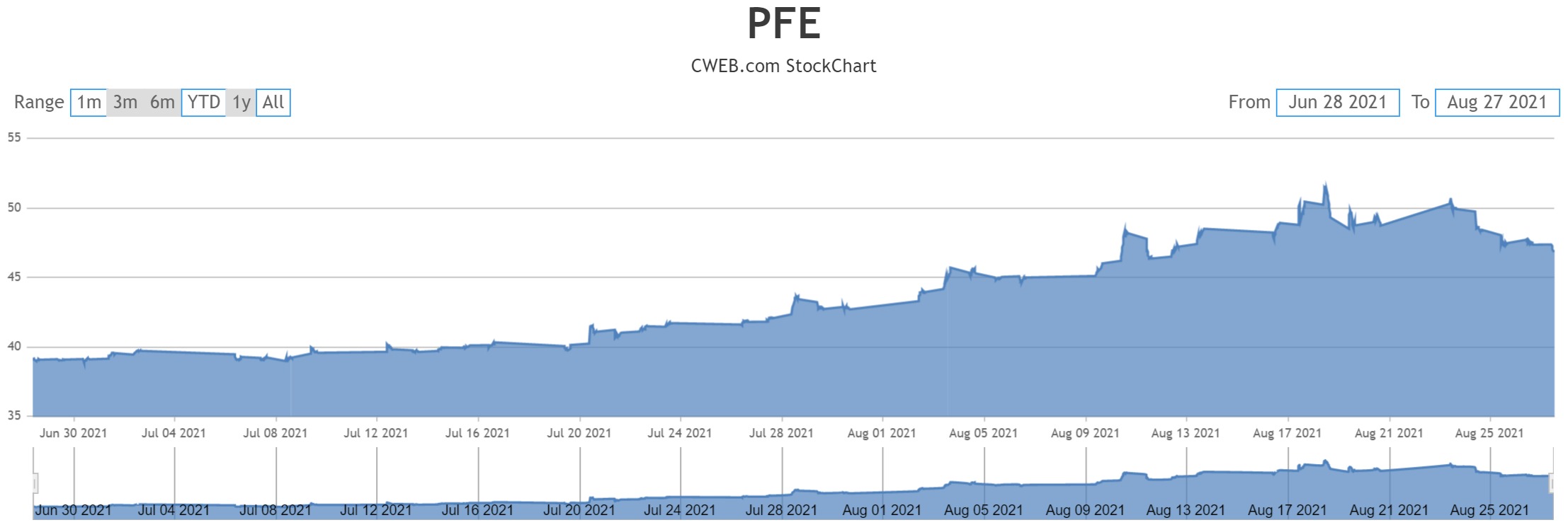
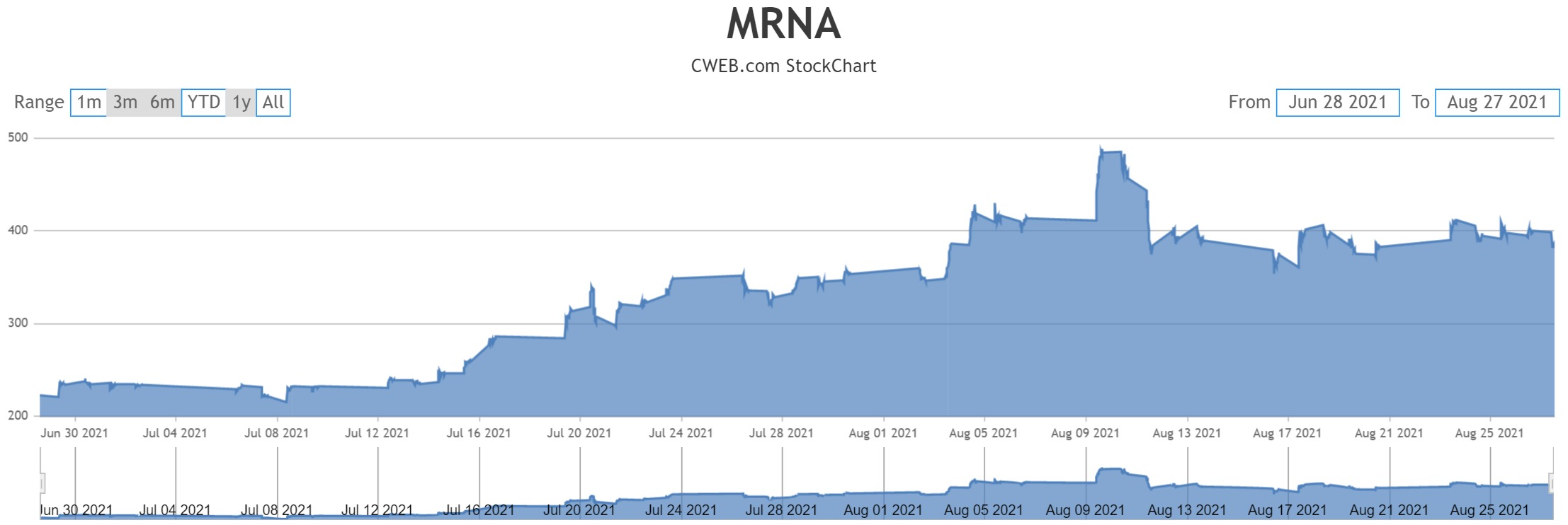
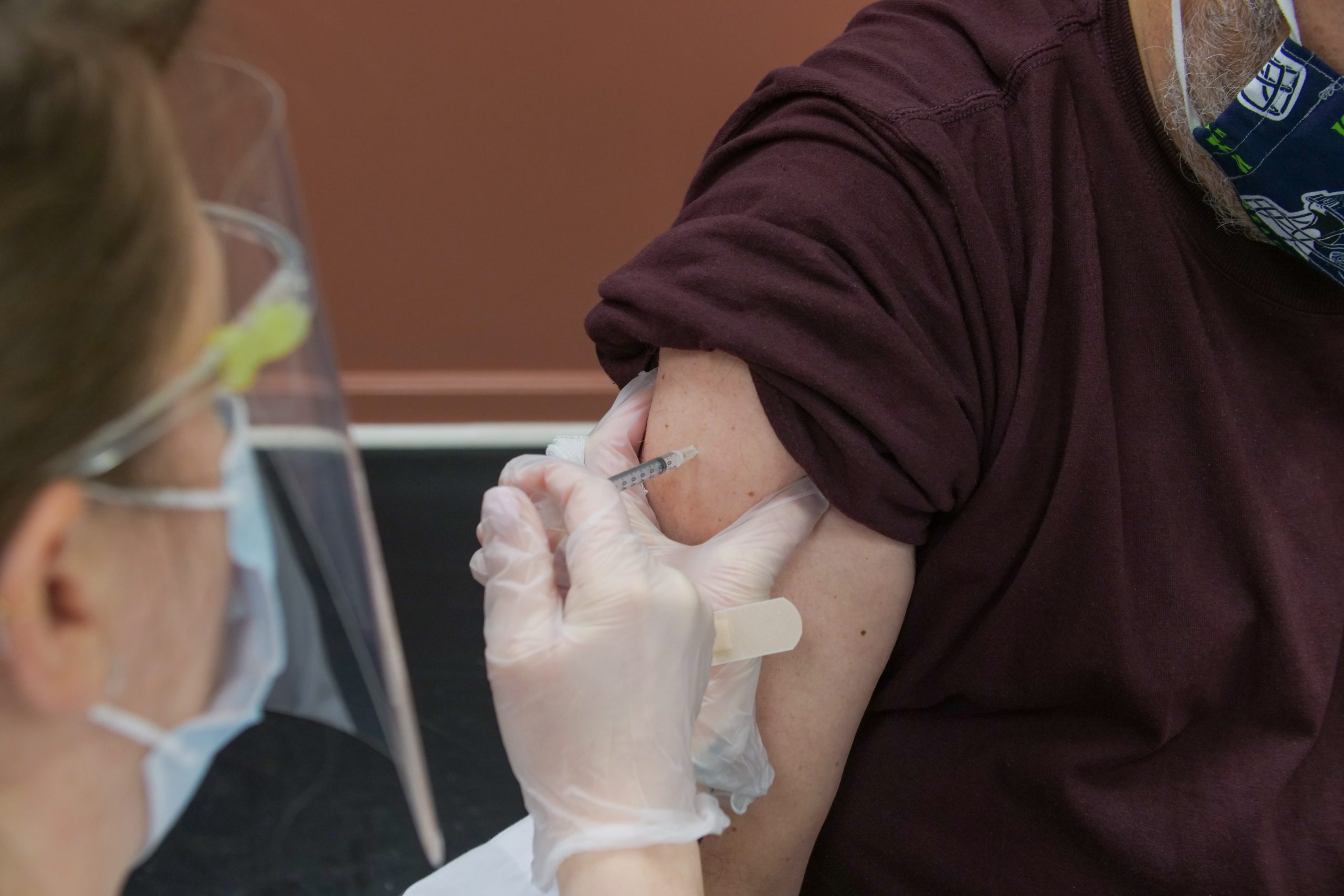
Amid all these cases, a person familiar with the matter told the Wall Street Journal those booster shots for all the three vaccines: Pfizer-BioNTech, Moderna and Johnson & Johnson could be given after six months.
Last week, U.S. officials said that booster shots will be given to all by mid-September, eight months after the second dose. However, after reviewing the data provided by the major vaccine manufacturers, and studies conducted in other countries, health authorities are most likely to recommend the booster shots after six months.
Studies have shown that the immunocompromised require booster shots as they have few or negligible antibodies after two doses. However, the number of antibodies increases after the third or booster shots.
Studies have also shown that the efficiency of the vaccine decreases after six months and this could be the main reason why the government is thinking of giving the third dose or booster shot after six and not eight months.
Two weeks ago, the Food and Drug Administration (FDA) had given the Pfizer-BioNTech and Moderna third dose or booster shots emergency use authorization so that those who have weak immunity could get the third dose. Now, it may soon be available for all, six months after people have taken the second of Pfizer-BioNtech or Moderna or six months after they have taken the single dose Johnson & Johnson single dose vaccine.